Investigating a New Approach for Patients With Devastating Lung Disease
Fibrotic lung diseases, also known as interstitial lung diseases (ILDs), are a group of more than 200 diverse diseases that cause progressive, abnormal scarring in the lungs. Over time, this scar tissue reduces the ability of the lungs to absorb oxygen and can lead to serious illness and death.
IPF
Idiopathic pulmonary fibrosis
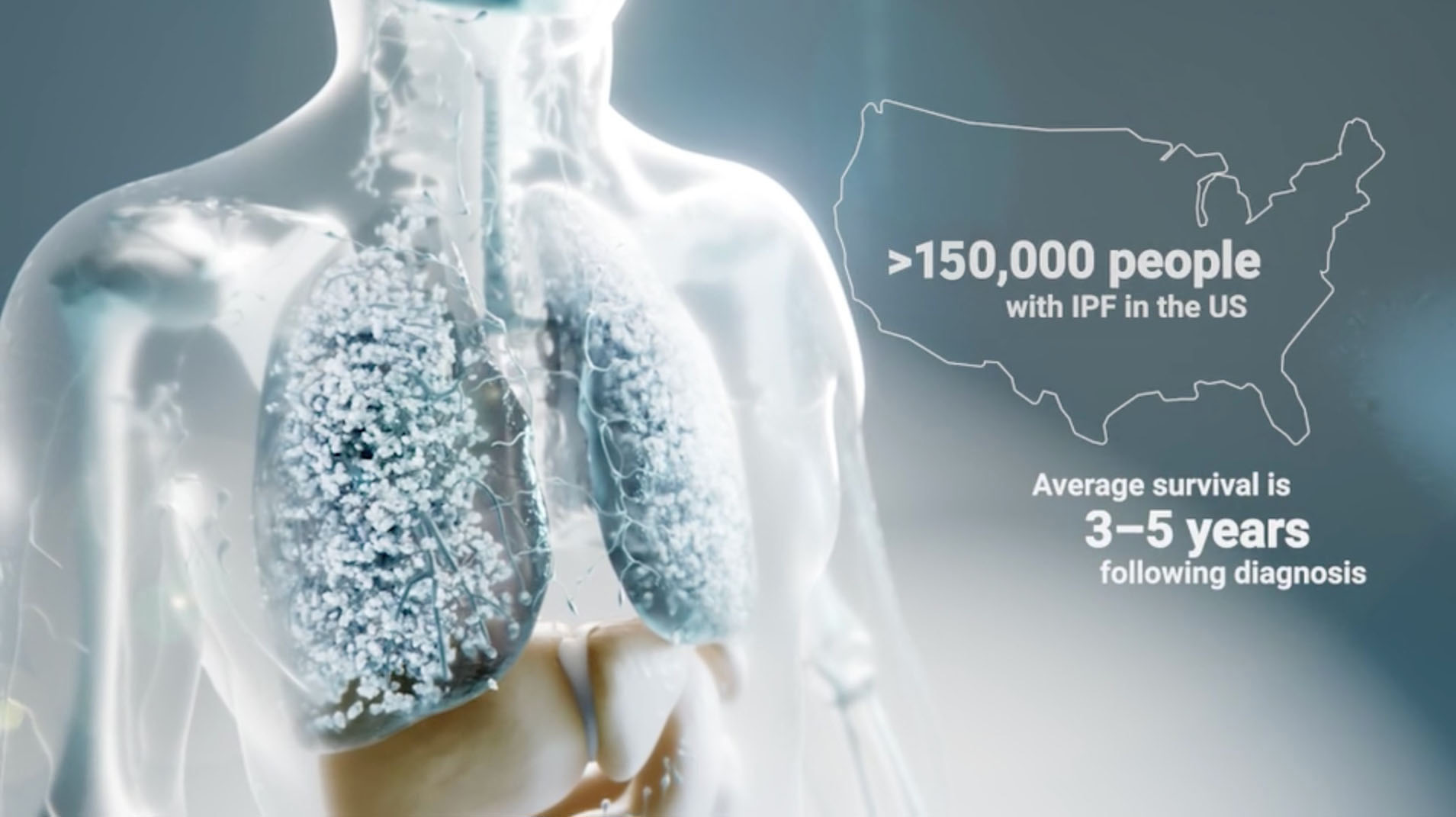
IPF is the most common type of ILD, affecting more than 150,000 adults in the United States. Although the exact cause of IPF is unknown, various environmental factors can deliver repeated injuries to lung cells that trigger abnormal wound healing processes and life-threatening lung scarring. IPF is a chronic disease with limited treatment options and a very poor prognosis: the average life expectancy is only three to five years after diagnosis.
Even individuals with IPF who are treated with the current standard of care medications suffer from decreased quality of life and limited longevity. Current standard of care does not address the underlying cause of IPF – it may minimally slow the decline of lung function, but does not stop or reverse it, and is associated with tolerability issues that limit its long-term use in most patients.
“Patients with IPF need a transformational therapy – one that changes the expectation for treatment from slowing the inevitable disease progression to reversing lung fibrosis and improving lung function.”
John Hood, PhD
Co-Founder, CEO and Chairman
Endeavor’s Disruptive Approach
Endeavor BioMedicines is determined to change the relentless progression of IPF. Our approach has demonstrated potential to block a cellular wound-healing pathway known as Hedgehog (Hh) that is abnormally activated in IPF and causes the buildup of scar tissue in lungs. Our lead compound is taladegib (ENV-101), an Hh signaling pathway inhibitor that has demonstrated compelling proof of concept showing an improvement in lung function, an increase in total lung capacity and reduction of key measures of lung fibrosis in patients with IPF. We’re currently enrolling patients in a global Phase 2b study called WHISTLE-PF that will further evaluate the safety and efficacy of taladegib in patients with IPF.