ENV-501: New Therapeutic Possibilities for HER3-Positive Cancers
ENV-501 is an optimized next-generation anti-human epidermal growth factor receptor 3 antibody-drug conjugate (HER3 ADC). Endeavor has the exclusive worldwide rights to ENV-501 and is developing the compound as a safer and more effective alternative to earlier generation HER3 ADCs.
About Antibody-Drug Conjugates (ADCs)
ADCs are a class of promising new cancer-therapy that are designed to very specifically target and kill cancer cells. ADCs consist of a monoclonal antibody attached by a tightly bound linker to a toxin – also known as a payload. Once infused, the monoclonal antibody seeks out tumor cells to kill them by delivering and releasing their toxic payload inside the cells.
HER3-positive cancers are a broad group of solid tumor cancers associated with poor prognosis and drug resistance, making them an important target for new treatments. Previous clinical research has demonstrated that the HER3 protein is a valid target for ADCs; however, improvements to ADC design are needed to maximize their effectiveness and minimize their toxicity to healthy cells.
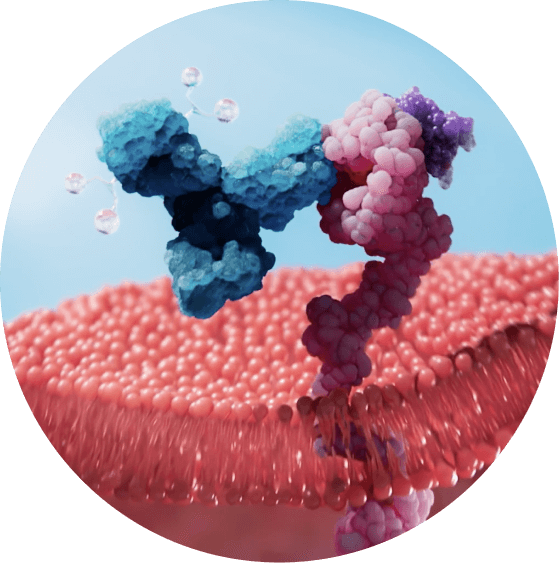
ENV-501 incorporates multiple proprietary, chemical and genetic features engineered to overcome specificity, tolerability and safety issues associated with earlier generation anti-HER3 ADCs:
- Monoclonal antibody optimized to bind very specifically to HER3 on tumor cells
- Additional improvements to the antibody lead to reduced uptake by non-tumor cells such as immune cells
- A hydrophilic linker offsets the hydrophobic toxin to reduce the likelihood of payload being released in the circulation where it could damage healthy tissue
ENV-501’s payload is clinically validated. After it binds to HER3 on tumor cells, ENV-501 is rapidly internalized into the cells and releases its payload, which works by disrupting DNA replication in tumor cells and ultimately results in tumor cell death.
“ENV-501 has the potential to be a leading next-generation antibody-drug conjugate that addresses significant unmet need among a large number of patients with multiple tumor types.”
John Hood, PhD
Co-Founder, CEO and Chairman
Evaluating ENV-501
Endeavor intends to initiate a Phase 1 dose-escalation trial of ENV-501 in patients with cancers that overexpress HER3.
